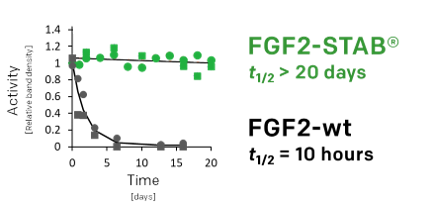Based on fibroblast growth factor 2 (FGF2, bFGF)
- ability to induce cell proliferation and maintain cells in their undifferentiated state
- essential component in both stem cell culture and cultured meat media
- native FGF2 is intrinsically unstable and therefore requires to be added to the media often and in high concentrations
FGF2-STAB® MEAT
- Engineered protein with improved stability and longevity
- Cost-effective as lower concentration is required in the media
- Patented molecule (WO2017089016A1)
- Called FGF2-G3 in publications
- Suitable for cultured meat applications – possibility of reformulation according to the customer’s specs
- REGULATORY: FGF2-STAB is considered a processing aid with no regulatory requirements limiting its use in cultured meat production
- Available in both bovine/porcine form, fish forms (salmon, eel and tuna/grouper) and human form
- Option of supply/collaboration/license agreements
COMPETITIVE ADVANTAGE
- Much lower dosage needed (5- to 20-times)
- Longer half-life (50-times)
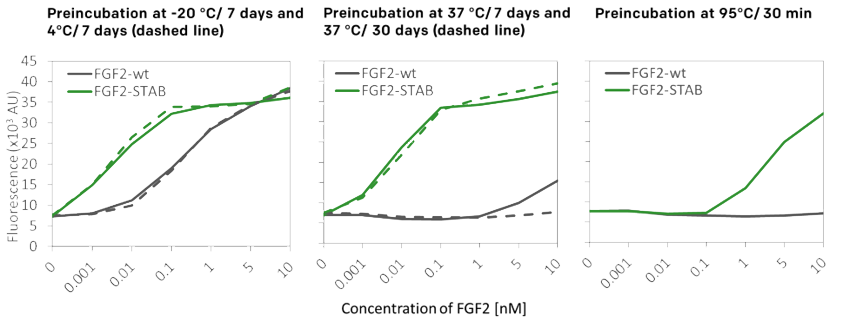
- Fully retained biological activity
- No need for stabilizing additives
- Animal-free product
01
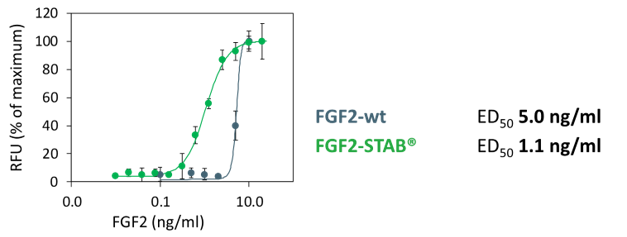
Lower dosage required
Measured by NIH/3T3 fibroblast cell proliferation
* 5-times lower dosage required, but up to 20-times lower dosage required if used in B8 media formulation (Kuo et al., 2020)
02
50-times longer half-life
03
Enhanced stability in cell proliferation assay
04
Temperature stability enhanced by 19°C
VISION
- FGF2-STAB® Meat is ready to become a game changer in the cultured meat space as a highly effective and cost-efficient product with global traction
- FGF2 is a key component of cultured meat media representing around 60% of media cost when wild-type FGF2 is used
- Scale-up of cultured meat production will require significant reduction of media cost
- According to the regulatory agencies, growth factors will be considered processing aid in the cultured meat process – therefore no regulatory requirements are limiting the use of our engineered molecule
Possibility of reformulation according to customers’ needs
Open to agreements for supply / collaborations / licenses
Literature
Dvorak P, Bednar D, Vanacek P, et al. Computer-assisted engineering of hyperstable fibroblast growth factor 2. Biotechnol Bioeng. 2018;115(4):850-862. doi:10.1002/bit.26531
Koledova Z, Sumbal J, Rabata A, et al. Fibroblast Growth Factor 2 Protein Stability Provides Decreased Dependence on Heparin for Induction of FGFR Signaling and Alters ERK Signaling Dynamics. Front Cell Dev Biol. 2019;7:331. Published 2019 Dec 12. doi:10.3389/fcell.2019.00331
Kuo HH, Gao X, DeKeyser JM, et al. Negligible-Cost and Weekend-Free Chemically Defined Human iPSC Culture. Stem Cell Reports. 2020;14(2):256-270. doi:10.1016/j.stemcr.2019.12.007
OUR CUSTOMERS